Have you ever walked into your house and immediately known that your mom was cooking your favorite pasta, even though you were still in the hallway? Or have you wondered why a drop of blue ink quickly turns a whole glass of water blue without you even stirring it?
These everyday “mysteries” are actually our first introduction to the world of Chemistry. Everything we see, touch, or smell—from the stars in the sky to the water we drink and the air we breathe—is made up of “matter.” Simply put, matter is anything that has mass and occupies space. If it takes up room and you can weigh it, it’s matter.
In these matter in our surroundings notes class 9, we are going to break down the microscopic world into simple ideas that will help you ace your 2026 exams.
The Physical Nature of Matter
If you look at a block of wood, it looks like one solid, continuous piece. But if you were to zoom in with a super-powered microscope, you’d see that it’s actually made of billions of tiny particles.
How Tiny are These Particles?
To give you an idea of the scale, imagine taking a single drop of water and trying to count the particles inside. You couldn’t. There are about 1.67 \times 10^{21} molecules in just one drop! These particles are small beyond our imagination.
The Personality of Particles
Particles aren’t just sitting there; they have specific “behaviors” that explain how the world works:
- They have space between them: When you mix sugar into tea, the sugar seems to disappear. It doesn’t vanish; the sugar particles simply park themselves in the tiny empty spaces between the water particles.
- They are always on the move: This is called Diffusion. Particles are restless. The smell of perfume reaches you because the scent particles are “dancing” and mixing with the air particles until they reach your nose.
- They attract each other: Think of particles like they are holding hands. In some materials, they hold on very tight (like in a piece of iron), and in others, the grip is very weak (like in the air).
Try This at Home: Take a glass of water and add a spoon of salt. Mark the water level. Stir it until the salt dissolves. You’ll notice the water level hasn’t risen! This proves that the salt particles moved into the existing spaces between the water molecules.
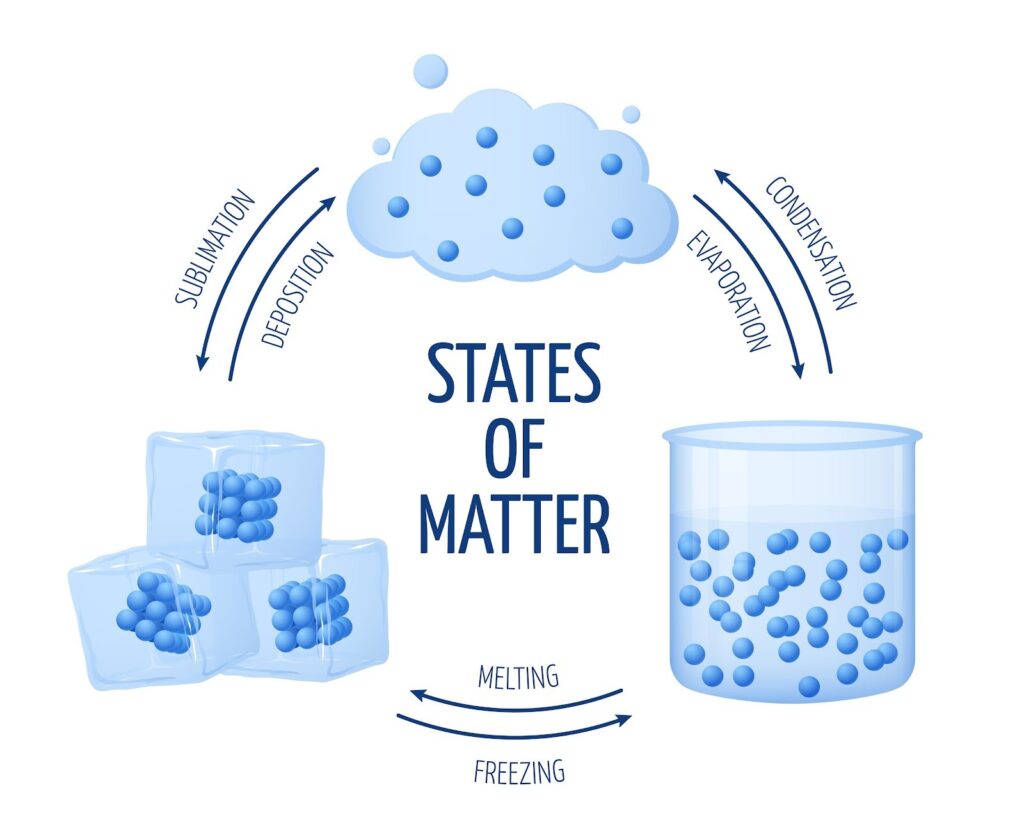
The Big Three: States of Matter
Matter doesn’t always look the same. Depending on how close the particles are and how fast they move, we categorize matter into three main states: Solid, Liquid, and Gas.
Comparing the States
| Feature | Solids | Liquids | Gases |
| Shape & Volume | Fixed shape and volume. | No fixed shape (takes the shape of the container) but fixed volume. | No fixed shape or volume. |
| Compressibility | Negligible (you can’t squeeze a stone). | Very low. | Highly compressible (think of CNG cylinders). |
| Diffusion | Extremely slow. | Faster than solids. | Very fast (fastest). |
| Particle Gap | Very little space. | Moderate |
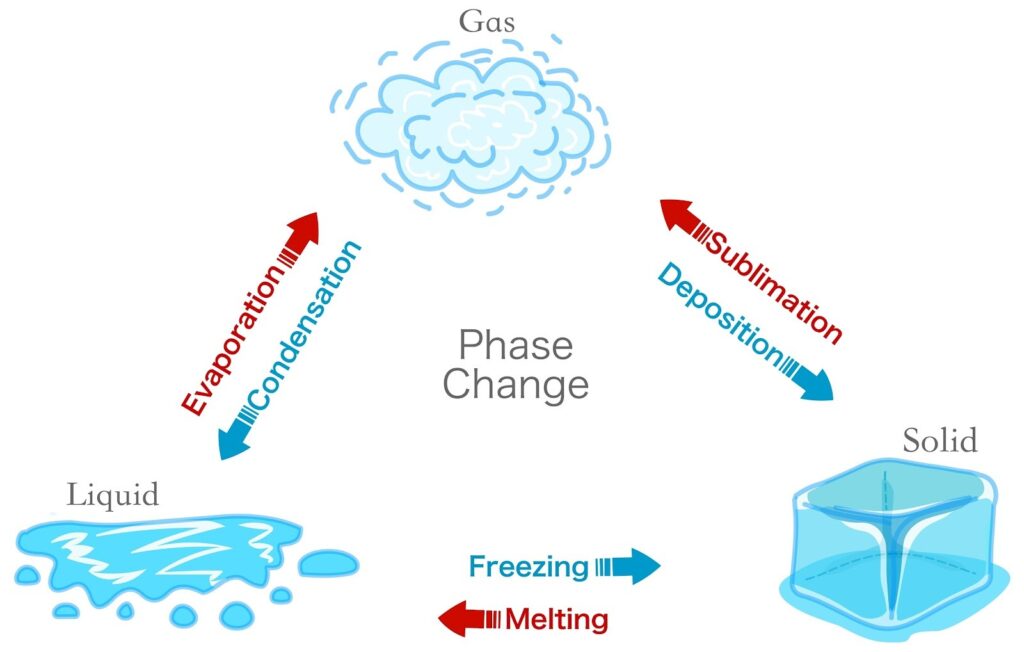
Can Matter Change its State?
The short answer is: Yes, absolutely. You see it every time you take an ice cube out of the freezer. Matter changes state by changing two things: Temperature or Pressure.
1. The Power of Heat (Temperature)
When we heat a solid, the particles start vibrating like they’ve had too much caffeine. Eventually, they break free from their fixed positions.
- Melting (Fusion): The temperature at which a solid turns into a liquid.
- Boiling (Vaporization): The temperature at which a liquid starts turning into a gas at atmospheric pressure.
The Mystery of “Latent Heat”
Have you noticed that when ice is melting, the temperature stays at 0°C until every bit of ice is gone, even though you’re still heating it? Where is that heat going? This is called Latent Heat (hidden heat). It’s being used to break the “hand-hold” (force of attraction) between particles rather than raising the temperature.
- Latent Heat of Fusion: The heat needed to change 1kg of solid to liquid.
- Latent Heat of Vaporization: The heat needed to change 1kg of liquid to gas.
2. The Power of Squeeze (Pressure)
If you take a gas and squeeze it (increase pressure) while cooling it down, the particles get so close that they turn into a liquid. This is how we get LPG (Liquefied Petroleum Gas) in our kitchens.
3. Skipping Steps: Sublimation
Some substances are “impatient.” They don’t want to become liquids.
- Sublimation: When a solid turns directly into a gas (like Camphor or Napthalene balls).
- Deposition: When a gas turns directly into a solid.
Evaporation: The “Cool” Concept
Many people confuse evaporation with boiling. Boiling happens at a specific temperature (100°C for water), but evaporation happens all the time, even at room temperature. It’s a surface phenomenon where the surface particles gain enough energy to fly away.
What makes things evaporate faster?
- Surface Area: Clothes dry faster when spread out than when bunched up.
- Temperature: A sunny day dries clothes faster than a cloudy one.
- Humidity: If the air is already full of water (like on a rainy day), evaporation slows down.
- Wind Speed: Ever notice how your hair dries faster under a fan?
Why does Evaporation cause cooling?
When liquid particles evaporate, they take energy from their surroundings. This leaves the surroundings cooler.
- Earthen Pots (Matka): Water seeps through tiny pores and evaporates, keeping the water inside chilled.
- Sweating: When we sweat, the moisture evaporates from our skin, taking body heat with it and cooling us down.
- Cotton Clothes: We wear cotton in summer because it absorbs sweat and exposes it to the air for easy evaporation.
Important Definitions & Units for Exams
To score well, you need to be precise with your units.
- Temperature Units: Scientists use Kelvin (K).
- To convert Celsius to Kelvin: K = °C + 273.
- Example: 0°C = 273 K.
- Dry Ice: This is solid carbon dioxide (CO_2). It’s called “dry” because it turns directly into gas without leaving any liquid behind.
- The “Other” States: While we focus on three, there are actually five. Plasma (found in stars and neon signs) and Bose-Einstein Condensate (created at super-low temperatures).
Important Questions for 2026 Exams
Based on previous years, here are the questions you should be ready for:
Q1: Why does a desert cooler cool better on a hot, dry day?
Answer: On a hot, dry day, the temperature is high and humidity is low. Both these factors increase the rate of evaporation of water, leading to a better cooling effect.
Q2: Convert 300 K to the Celsius scale.
Answer: Celsius = K – 273. So, 300 – 273 = 27°C.
Q3: Why do we feel cold when we put some acetone (nail polish remover) on our palm?
Answer: Acetone particles have a very low boiling point. They take energy from your palm to evaporate, causing your palm to feel cold.
Summary
- Matter is anything with mass and volume, made of tiny particles.
- Particles are always moving and have spaces between them.
- States of matter (Solid, Liquid, Gas) can be changed by temperature or pressure.
- Latent heat is the “hidden” energy used to change state without changing temperature.
- Evaporation is a surface process that causes a cooling effect.
Ready to get the full version?
If you found these notes helpful, you can grab the high-quality PDF version which includes extra diagrams and a solved question bank.


